FDA Reviews COVID-19 Vaccine Updates Amid New Variant Concerns
The FDA is considering updates to COVID-19 vaccines as a new variant drives case surges in Asia and is detected in U.S. travelers.
Subscribe to unlock this story
We really don't like cutting you off, but you've reached your monthly limit. At just $5/month, subscriptions are how we keep this project going. Start your free 7-day trial today!
Get StartedHave an account? Sign in
Overview
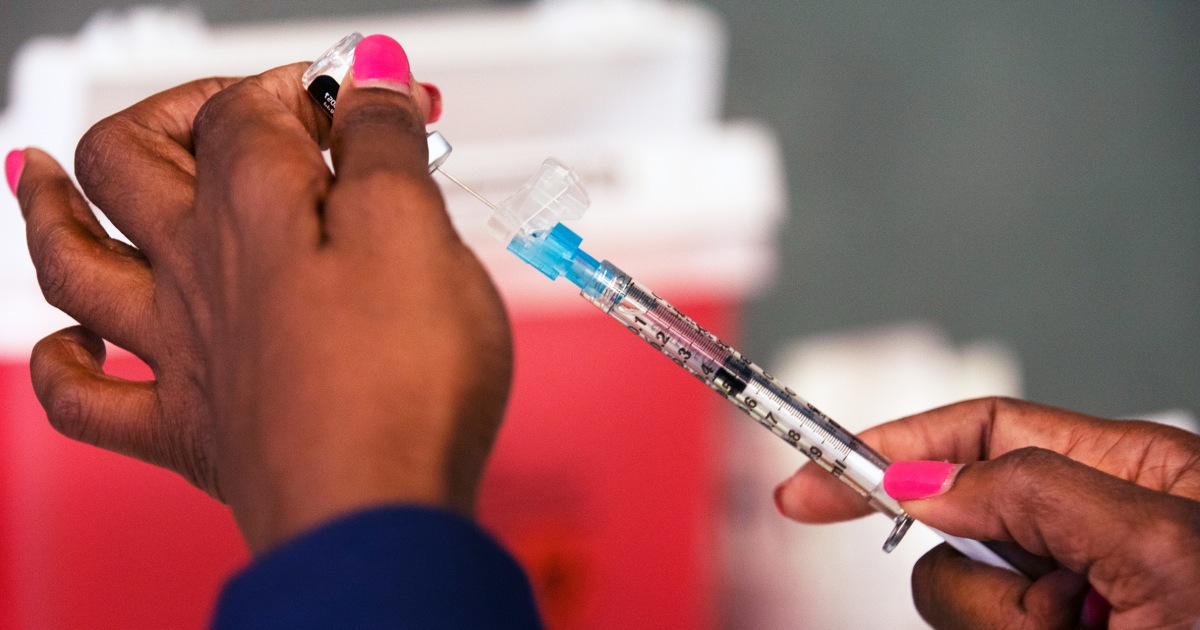
The FDA is reviewing COVID-19 vaccine recipes for the upcoming fall and winter, focusing on updates amid new guidelines limiting booster eligibility to seniors and high-risk individuals. This meeting is the first since the Trump administration's policy changes, raising concerns about vaccine access for healthy individuals under 65. New cases of the NB.1.8.1 variant, linked to a surge in Asia, have been reported among international travelers in the U.S. The CDC will provide recommendations in June, as COVID-19 continues to impact public health significantly, with thousands of deaths reported since October.
Report issue

Read both sides in 5 minutes each day
Analysis
Analysis unavailable for this viewpoint.
Articles (10)
Center (4)
FAQ
The FDA is reviewing updates to COVID-19 vaccines to include a monovalent JN.1 lineage formula for the 2025-2026 season, which is based on recent vaccine effectiveness studies showing positive health outcomes with this strain update.
The FDA's recent policy changes limit COVID-19 vaccine recommendations to adults aged 65 and older and people at high risk, focusing on those groups due to risk-benefit considerations and to prioritize vaccine resources amid evolving pandemic circumstances.
The NB.1.8.1 variant is linked to a recent surge of COVID-19 cases in Asia and has been detected among international travelers in the United States, raising concerns about potential new waves of infection and prompting updates in vaccine strain selection.
The CDC is expected to provide its recommendations on updated COVID-19 vaccines in June, following the FDA's review and advisory meetings.
The 2024-2025 formula vaccines showed about 33% effectiveness against COVID-19-associated emergency department or urgent care visits among adults aged 18 and older, with slightly higher effectiveness around 45-46% for other age groups.
History
- 5M

 4 articles
4 articles
- 5M

 3 articles
3 articles