Scientists Create Fertilizable Human Eggs from Skin Cells in Lab
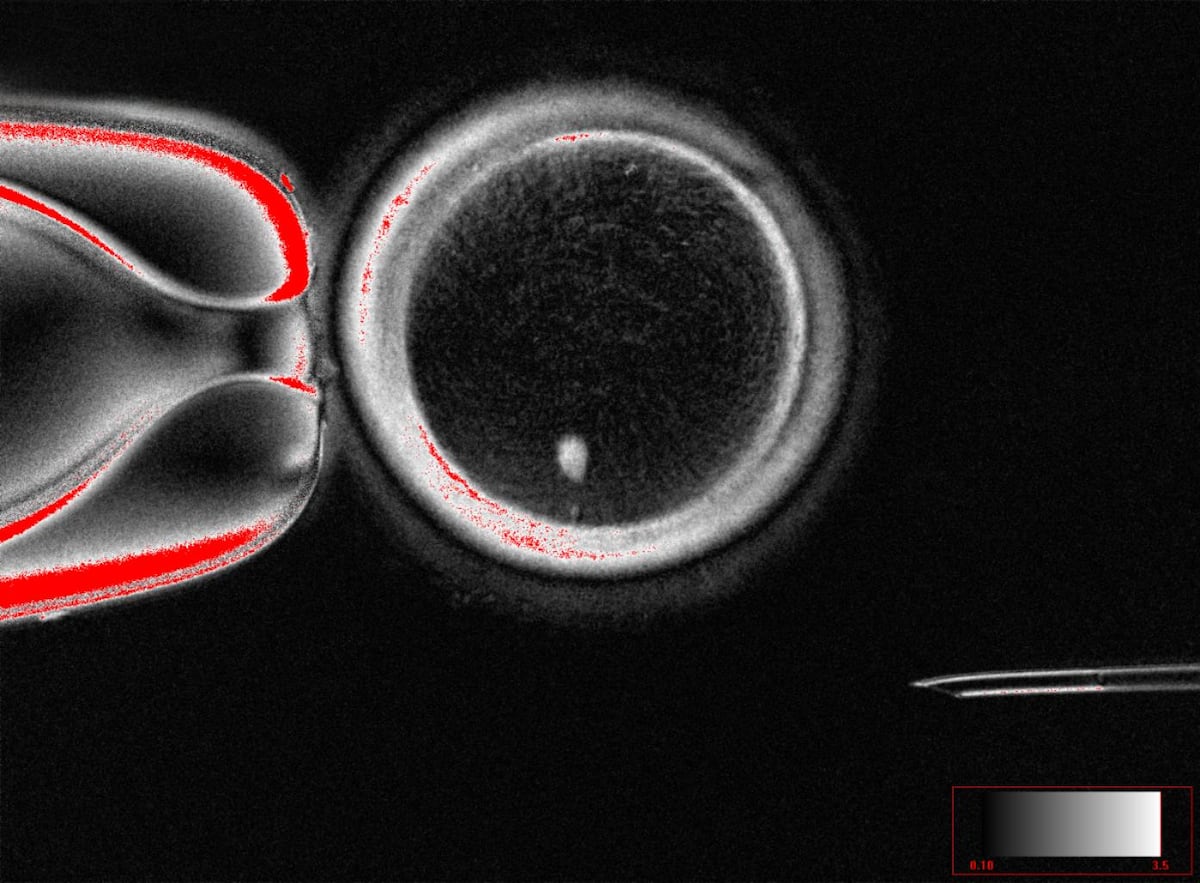
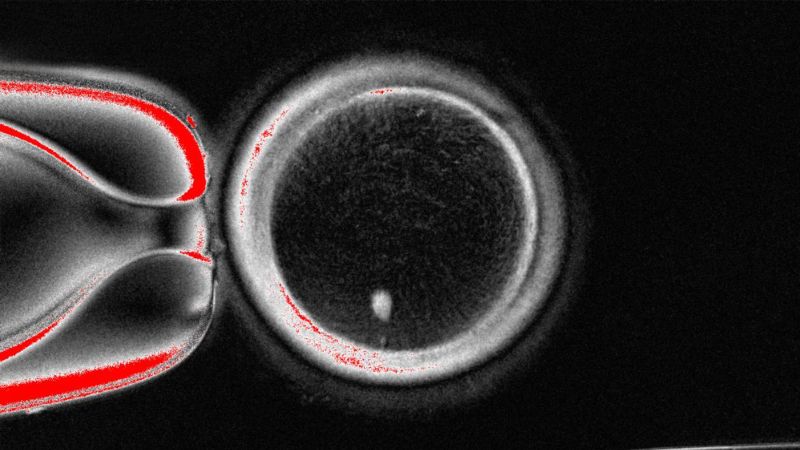
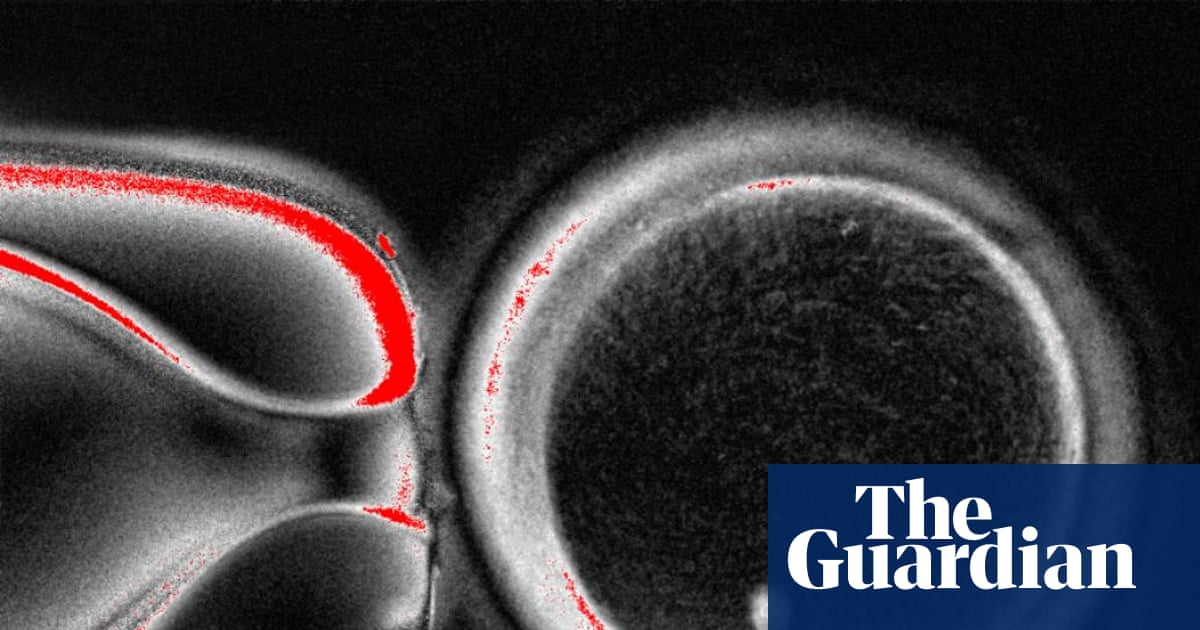
Scientists successfully created fertilizable human eggs from skin cells using somatic cell nuclear transfer. The resulting embryos, however, showed chromosomal abnormalities, making them unsuitable for implantation.
Subscribe to unlock this story
We really don't like cutting you off, but you've reached your monthly limit. At just $5/month, subscriptions are how we keep this project going. Start your free 7-day trial today!
Get StartedHave an account? Sign in
Overview
- Scientists successfully created fertilizable human eggs from skin cells in a groundbreaking laboratory experiment, offering potential new avenues for infertility treatment affecting millions globally.
- The research involved using somatic cell nuclear transfer (SCNT), a technique previously employed to clone Dolly the sheep, to reprogram skin cells into functional eggs.
- Researchers successfully created 82 functional human eggs, which were then fertilized with sperm and developed into early-stage embryos, with about 9% of skin cells forming blastocysts.
- Despite the successful creation and fertilization, the resulting embryos exhibited significant chromosomal abnormalities, rendering them non-viable for healthy baby development or implantation.
- While praised as a breakthrough, scientists acknowledge that these lab-created eggs may differ from naturally produced ones, and further research is needed before clinical application.
Report issue

Read both sides in 5 minutes each day
Analysis
Center-leaning sources cover this story neutrally, focusing on the scientific achievement and its potential. They describe the complex process of creating early-stage human embryos from skin cells, highlighting both the promise for infertility and same-sex couples, alongside the significant challenges and need for further refinement. The reporting maintains an objective tone, presenting facts without overt evaluative language.
Articles (9)
Center (4)
FAQ
Somatic cell nuclear transfer (SCNT) is a technique where the nucleus of a somatic cell, such as a skin cell, is transferred into an egg cell that has had its own nucleus removed. This reprograms the somatic nucleus to behave like an egg nucleus, allowing the creation of functional eggs from non-reproductive cells. In the reported study, SCNT was used to create 82 fertilizable human eggs from skin cells, which were then fertilized by sperm and developed into early-stage embryos.[1]
The embryos showed significant chromosomal abnormalities because the eggs created by SCNT did not undergo normal chromosome recombination during meiosis, resulting in eggs with incorrect numbers of chromosomes. This led to fewer than 10% of the fertilized eggs developing into blastocysts, and all embryos were non-viable due to aneuploidy and other genetic instability issues common in SCNT-derived embryos.
SCNT for human reproduction raises concerns about unknown physical harms, long-term safety risks, genetic anomalies, psychosocial harms such as violations of privacy and autonomy, potential harm to cloned individuals' well-being, and risks of adverse societal effects including eugenics and discrimination. Due to these concerns and the unresolved risks, reproductive cloning using SCNT is not endorsed by the medical profession or society.[2]
Key improvements include ensuring the created eggs have the correct number of chromosomes (23) with proper recombination, overcoming chromosomal abnormalities, and demonstrating safety and viability of resulting embryos. Additionally, more research is required to address the differences between these lab-created eggs and natural eggs, and to ensure clinical safety and ethical acceptability before they can be used in reproductive treatments.[3]
While SCNT has been successfully used in animals to clone species like Dolly the sheep, it often results in health problems including chromosomal abnormalities, lower reproductive success, and organ failures. Human application faces additional challenges due to higher rates of aneuploidies, limited availability of healthy oocytes, genetic and epigenetic instability, and differences in cell cycle physiology. These challenges make producing viable human blastocysts and embryos via SCNT very difficult and require further technical improvements.
History
- This story does not have any previous versions.